Contents
Training
CITI Training
Before you can run subjects or interact with data that hasn't been deidentified, you must complete Human Subjects Protection Program training through CITI, and be added to the relevant protocol(s).
Initial CITI Certification
CITI Recertification
If you have any questions about registering for CITI or completing this training, please contact Kelly Unsworth at (585) 275-5244 or by email at kelly_unsworth@urmc.rochester.edu.
Once you have completed the CITI Training and received your confirmation letter, contact the study coordinate / your supervisor to be added to the protocol. They will submit an amendment to add you to the protocol as soon as possible, and once you have been added then you can run subjects and interact with data.
Adding Personal to Protocol
- Log into IRB Click, find correct study
- Submit a MOD choose “study team member information”
- Description of modification: adding new personnel (list names and what they will be doing).
- Go to personnel page and add the new personnel.
The guideline for investigators is located on the web: http://www.rochester.edu/ohsp/rsrb/training/clickIRBTraining.html
Investigator Training
Check the Office for Human Subject Protection's training index for more training opportunities, both online and in-person.
Subject Recruitment
In general, each study has a physical flyer that can be posted on the River Campus and at the Medical Center, and a digital advertisement that can be posted on Craigslist and Facebook. Always get the most recent forms directly from Click IRB, and ensure you follow the recruitment method outlined in that specific IRB.
Kathleen Corser maintains the BCS department's human subject list.
Responses to New Subjects
When a potential new subject contacts us, we should provide them with information about the research study:
- All subjects must be at least 18 years old
- All subjects must be able to speak and read English
- Some studies require no glasses or contacts
If there is currently a waiting list, also say something like:
“At the moment, we have no available participant slots. But if you meet the above criteria and are going to be available later in the semester, we will add your email to our database and contact you as soon as a new study starts.”
Subject / Visitor Parking
Subjects will be allowed to park in the Library Lot for the duration of their visit with the lab. The code is 100606# (make sure that the pound sign is included). Do not share this code with anyone outside of the AP Lab.
Old Info Below This Line
If subjects or other visitors need parking, we will be able to request that accommodation on a case-by-case basis. Just let the lab manager know at least 24 hours in advance, and they will set it up on the Visitor and Special Event Parking Reservations site. The information that is needed is:
- The date
- The guest name
- Estimated time of arrival
- Whose study they're coming in for (or, in cases of non-subject visitors, who they are here to see)
New Subject Demo Session Outline
Note: You must be approved on a protocol in order to work with subjects on that protocol.
All forms are available on clickIRB. Digital consent forms are available on redcap for select IRB protocols.
- Tell them what to expect (including what a bite-bar is - MAKE SURE THEY CAN USE A BITE BAR)
- Screening form: Verbal consent to screening
- Be sure to sign and date it right away!*
- Visual Acuity Test:
Try the 20/20 line first; if they miss 2 or more, go up to the 20/25 line
If they miss 2 or more on the 20/25 line, they have failed the screening
If they get all correct, keep moving down the chart until they miss 2
Record their result on the demographic form (example result: 20/15-2 means they missed 2 on the 20/15 line)
- Consent form (on paper or redcap) and lab demographics form (on redcap)
- Be sure to sign and date it right away!*
- Assign them a subject # from the list, write it on the demographics form and on the numbers log
- Check if the Eye Tracker works on them with the subject on a chin rest
Explain in general what is going on
Explain the buttons on the game pad (Start, Top Right, Top Left)
Explain the calibration procedures
“Follow my finger" test
- If tracking went well, make bite bar imprint (see below)
- Schedule first session
- Payment (see below)
* You may only sign consent forms if you have been approved on the protocol. Please see the Study Personnel page if you don't know whether or not you're on a protocol.
E-Screening and E-Consent
- Initiate a zoom meeting with the subject.
- Read the e-screening script - be sure to sign and date right away!
Complete the e-screening at https://www.zeiss.com/vision-care/us/better-vision/vision-screening.html (need more details here)
If the subject has passed the e-screening, create a record for them on RedCap and ask their "favorite color" which will be their verification key.
In their RedCap entry, go to the Consent Security Verification survey. Enter their verification key and save the form.
Select the instrument with the name of the protocol you want to consent on. Scroll to the bottom to entries "Study Staff User". Verify that your name and the current date and time are listed in the two fields. Select Save & Stay from the top right.
- Under the Survey Options at the top of the page, select "Compose survey invitation" to send the consent documents via email. (You could also select to open the survey if the subject is physically present.)
- Send the demographics form similarly via e-mail.
If new e-consent forms are approved in clickIRB, request that Research and Academic IT modify the survey on RedCap via this link: https://redcap.urmc.rochester.edu/redcap/surveys/?s=K4FL8KKDKY
Existing Subject, New Protocol
- Inform subject about any differences in the studies
- Ask for their permission to use their screening results from the first round, OR screen them again if they/you prefer
- Give them the new consent form to read and sign
- Assign them a new number
- No need to redo the demographic form, just put the new number on their existing form
- Proceed with the rest of the study
- Have them bring the new forms to the lab manager
Bite Bars
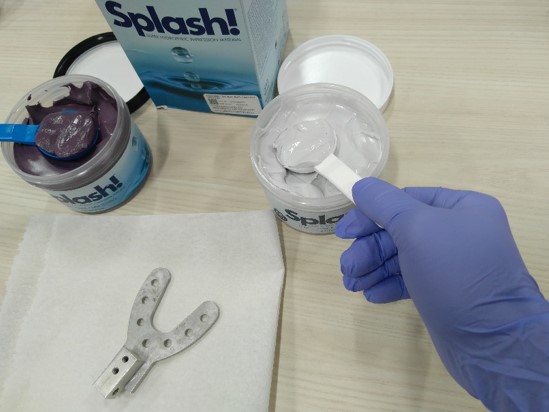
If the tracking is good, then we make a bite bar:
- Explain to the participant the purpose, steps and maintenance.
Put on gloves.
- Get a bite bar skeleton of a good size, and put it on a clean paper.
- Get one scoop of putty base (purple) with the blue scoop, and one scoop of putty catalyst (white) with the white scoop.
- Mix the putty well and divide it into two even parts, then attach them to each side of the skeleton and seal them together.
- Do this quickly, so that the putty does not become concrete.
- Ask the participant to bite on the bite bar.
- Do not bite too deep so that there is enough room for mounting.
- Make sure the bite bar is pointing straight forward; use the mirror!
- Do not bite too hard so that the teeth do not touch the metal.
- After ~1 min, take the bite bar out and put it in a bowl for sterilization and rinse.
- Leave the bite bar for more than 15min before using it.
Clean up after the subject
Place the bite bar in a bowl with Listerine (2 pumps is good) and top it off with water. Place the subject’s baggie over it (perhaps with a paper towel) so that we don’t mix up bite bars if there are more than one out at a time. After some time (20 minutes to a day) dry off the bite bar, wrap it in a paper towel, place it into the bag and put the bag in the appropriate drawer.
Use the rubbing alcohol to wipe down surfaces in the experiment room that subjects usually touch – definitely wipe off the head holder and maybe also the joy pad.
Payment
Subjects are paid from either Michele's or Martina's R01 grant. The orange binder in the lab manager's office contains the necessary forms: the F-6 forms (the summary sheet), the F4 forms for check requests (make sure you have the correct one **see below**) and the receipt book.
For tax purposes, we have to distinguish whether the participant is a US citizen/permanent resident or not.
If your subject IS a US citizen or permanent resident fill out:
- Petty Cash form:
- The important parts are:
- The date
- Subject signature
- Purpose: "Vision research subj. fee"
- The amount
- Acct Key: "A" (Michele's R01) or "B" (Martina's R01)
- The important parts are:
- Receipt:
- The important parts are:
- The date
- Subject printed name
- Subject non-University address (unless that's all they have)
- The amount
- For: "Vision Research"
- Acct Key: matching the petty cash form
- Your signature AND the subjects signature
- The important parts are:
- Payment:
- Give subjects the appropriate amount of money from the envelope
- The keys to the drawer are hanging on the key hook by MAC's desk
When you see that we're running low on money, take the box to the Bursar's Office to be refilled. See Replenishing Petty Cash for more details.
***IMPORTANT**** if the amount that you are paying a subject at one time over $275 you need to fill out a F4 form that is FOR A US CITIZEN (ask SJ or Michele Schultz for the form) AND W9 from F4 form for US citizen.
If your subject is NOT a US citizen or permanent resident:
1. F4 form for Non-US citizen/permanent resident:
Make sure it is specifically for a Non-US citizen. The F4 form for a Non-US citizen differs from the F4 form for a US citizen because the boxes on the top half of the first page are checked off differently; otherwise it is the same (ask Michele Schultz if you need help distinguishing the forms) F4 form for NON US citizen
- Pre-filled out forms are available in the orange binder (where is a digital copy for the future?)
- put the EMAIL ADDRESS of subject (this is how accounting contacts the subject)
The grant number; it is under as GR and is the same as the petty cash summary sheet grant numbers (also listed here). One check request can have have more that one grant on it.
- Payee type, payee, and payee address
- total amount paid (with subcategories if applicable)
- the person who is in charge of replenishing the petty cash also needs to bring the F4 form to be approved by Michele Schultz
- If you need the check processed quickly let the person who is doing the petty cash know asap.
- The F4 needs to be submitted with the signed receipts from each session
*** make sure that the subject is aware that they won't get the money for two weeks from when accounting processes the check
Data Management: REDCap
REDCap https://redcap.urmc.rochester.edu/redcap/ is a web application for building and managing online surveys and databases. It allows us to securely store demographic and study involvement information for our subjects; we have approval from the IRB to do so.
Right now, there is only one project for the Active Perception Lab subjects. It is called "APLab Subject Demographics"; it contains a demographics survey, which we can either manually fill out or send out to subjects, and a database for which protocols to count their involvement in.
Log in and select the "APLab Subject Demographics" project.
To add a new records or edit an existing one, click "Add / Edit Records" in the left sidebar. Select the record that you want to edit, or click the "Add new record" button. Records are split into two instruments: Demographics and For Office Use. Click the status icon next to the instrument that you want to edit.
The Demographics instrument contains fields for all of the information that we want subjects to fill in for themselves on the paper survey form. Fill it in as they have filled it in. When you are finished, mark it as "Complete" and choose one of the save options; "Save & Go To Next Form" will take you directly to the "For Office Use" instrument.
The For Office Use instrument contains everything in the top box of the paper survey form, minus the subject number and plus a section for notes. Fill it in with the available information. When you are finished, mark it as "Unverified" and click "Save & Exit Form".
When you are finished entering a paper demographics form, mark that it has been entered into REDCap by writing "RC" followed by the date somewhere on the form. File it away.
The How to Use REDCap document contains more details and a picture-based guide.
If adding a new field to RedCap, checkout this video:
